Calcium Carbonate Reacts With Hydrochloric Acid
Calcium carbonate reacts with dilute hydrochloric acid to form calcium chloride water and carbon dioxide gas. When calcium carbonate reacts with hydrochloric acid the products are calcium chloride carbon dioxide gas and water.
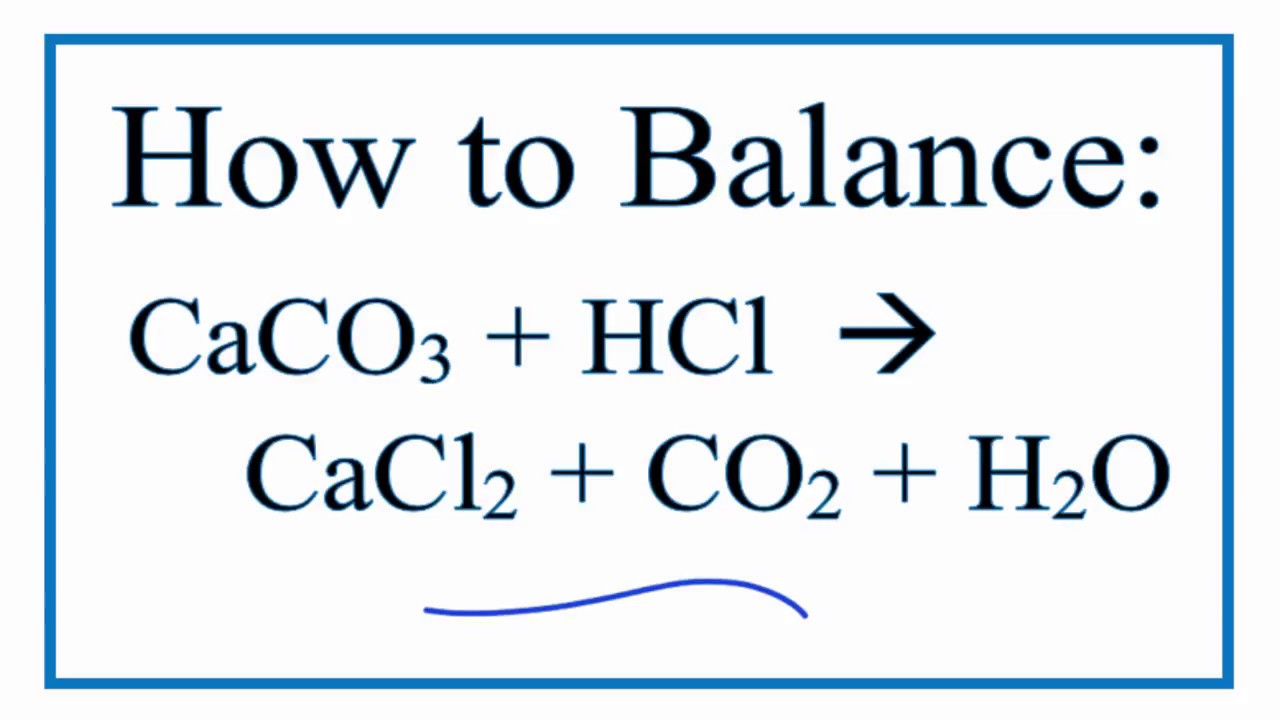
How To Balance Caco3 Hcl Cacl2 Co2 H2o Calcium Carbonate Hydrochloric Acid Youtube
Up to 256 cash back Used as an antacid the calcium carbonate acts a base where the carbonate anion reacts with the acid Hydrogen in stomach acid HCl in a neutralization.

. The balanced reaction is shown below. Calcium carbonate reacts with hydrochloric acid to form carbon dioxide gas. 130 g of magnesium chloride MgCl2 is dissolved in 300 mL of water.
What are the products of this reaction. CaCO3 2HCl -- CaCl2. Rates of Reaction Hydrochloric Acid Calcium Carbonate.
When hydrochloric acid comes into contact with calcium carbonate the following chemical reaction ensues. 2 HClaq CaCO3s CO2g H200 CaCl2aq In a reaction 75 g HCl is combined with 50 g CaCO3. Mobsile681 mobsile681 4 minutes ago Chemistry.
Chemistry Chemical Reactions Double. Calcium carbonate reacts with. Formal Report Essay Example for Free.
21 Calcium carbonate reacts with. Experiment is to investigate the. A Calculate the concentration of the solution.
Analysis of Calcium Carbonate Tablets emich edu. Carbonates calcium carbonate reacts with acidic reaction that is responsible for limestone fizzing when dilute hydrochloric acid is placed on its surface. Calcium carbonate reacts with hydrochloric acid as follows.
Here is the unbalanced equation. Hydrochloric acid reacts with calcium carbonate to produce calcium chloride carbon dioxide and water. One way of following the rate of reaction at which it reacts is to measure the volume of carbon dioxide.
21 Calcium carbonate reacts with hydrochloric acid to form carbon dioxide. A What is the. C a C O 3 2 H C l C a C l 2 H 2 O C O 2 When C O 2 is passed.
Calcium carbonate reacts with hydrochloric acid in a double-replacement reaction. In conclusion the net ionic equation for the reaction between calcium carbonate and hydrochloric acid is CaCO3 solid plus two H aqueous react to form Ca2 aqueous plus. CaCO3 2HCl CaCl CO2 H2O which provides acid neutralization.
Asked Jun 4 2020 in Chemistry by Joshua Mwanza.

Doc What Happens If Calcium Carbonate Reacts With Hydrochloric Acid Edwin Lee Academia Edu

A Substitute Formulae For Names And Balance The Following Equation Calcium Carbonate Reacts Youtube

Calcium Carbonate Reacts With Aqueous Hcl To Give Cacl2 And Co2 According To The Reaction Caco3 S 2hcl Aq Cacl2 Aq Co2 G H2o L What Mass Of Caco3
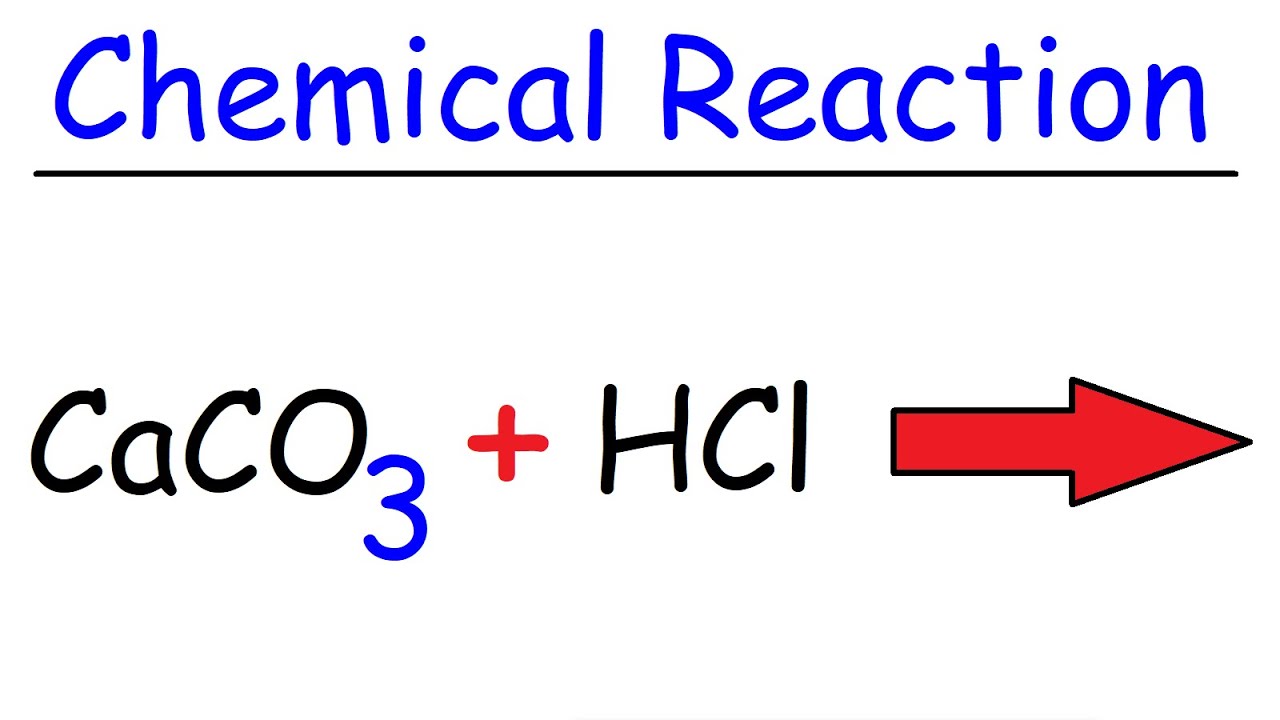
Caco3 Hcl Calcium Carbonate Hydrochloric Acid Youtube

Write A Balanced Chemical Equation For The Reaction Of Calcium Carbonate And Dil Hydrochloric Acid

Question Video Calculating The Average Rate Of Reaction Of Hydrochloric Acid With Calcium Carbonate Nagwa
0 Response to "Calcium Carbonate Reacts With Hydrochloric Acid"
Post a Comment